
Support Team
Feedback:
support@nextpcb.comThe demand for ventilators in countries around the world is now growing rapidly due to the impact of coronavirus. Because hospitals need ventilators to help patients keep breathing. However, hospitals and health departments said that the supply of ventilators could not satisfy the situation of the rising number of cases, which prompted manufacturers to produce more ventilators urgently.
According to the reports from foreign media, the United States recently signed a new contract with the manufacturer worth more than 2.5 billion US dollars. It is estimated that by May 8, General Electric, Medtronic, and other manufacturers plan to deliver 6,190 new ventilators to the US Strategic National Reserve. By June 1st, 29,510 ventilators will be delivered, and by the end of 2020, 137,431 new ventilators will be delivered.
At present, not only are traditional ventilator manufacturers making full use of their power to increase productivity, car manufacturers such as General Electric, Ford, Tesla, Lamborghini, etc. have also joined to manufacture ventilators. And even Foxconn has teamed up with Medtronic to produce ventilators at the Wisconsin plant in the United States.
But even so, it is still difficult to meet the demand for ventilators in the short term. Why cannot the production capacity of the ventilator be increased to a level that can meet the demand in one or two months after the Chinese manufacturing plants are fully used? Like the face masks and frontal temperature guns, we were in short supply at the beginning of the year, but the production capacity quickly kept up with the requirements.
Great demand for ventilator with low production capacity
According to public information, foreign companies currently dominate the ventilator market, such as Medtronic, Dräger, Vyaire Medical, Hamilton Medical, Getinge, and Philips. Among them, Hamilton Medical, Dräger, Getinge, and Beijing Yi'an share half of the global market.
The marketing director of the medical industry in ADI China, Peng Zhifeng said, the ventilator is a product category with little demand in the medical device product classification. There are usually a few non-invasive ventilators in the respiratory medicine department of ordinary hospitals, only ICU ward Equipped with a small number of invasive ventilators. Moreover, the ventilator is a durable product in the hospital. The general service life of a ventilator is more than six years, or even exceed ten years. Therefore, the annual production capacity of traditional ventilator manufacturers will not be very substantial.
In normal years, Philips's weekly production is about 1,000 units, Italy manufacturer Siare's weekly production is about 75 units, Germany manufacturer Dräger and Sweden manufacturer Getinge's weekly production are about 200 units, and Swiss Hamilton's weekly production is about 560 units. The total production capacity of these significant manufacturers is about 2035 units a week. Some organizations predict that there is a shortage of nearly one million ventilators around the world. It is hard to meet such a large amount of demand, even if the production capacity doubles.
High barriers in ventilators production
Unlike face masks and frontal temperature guns that can boost productivity quickly, the production capacity of the ventilator is challenging to break out soon because the production barriers of the ventilator are relatively high. Ventilators belong to Class II or Class III medical devices.
According to classification in the 2017 edition of the "Catalogue of Medical Devices" issued by the National Medical Products Administration (NMPA), home respiratory support equipment (non-life support) and sleep apnea treatment equipment belong to Class II medical devices, which are commonly used in the home. Therapeutic ventilator (life support), first-aid and transfer ventilator, high-frequency ventilator (HFV), home ventilator (life support) belong to Class III medical devices.
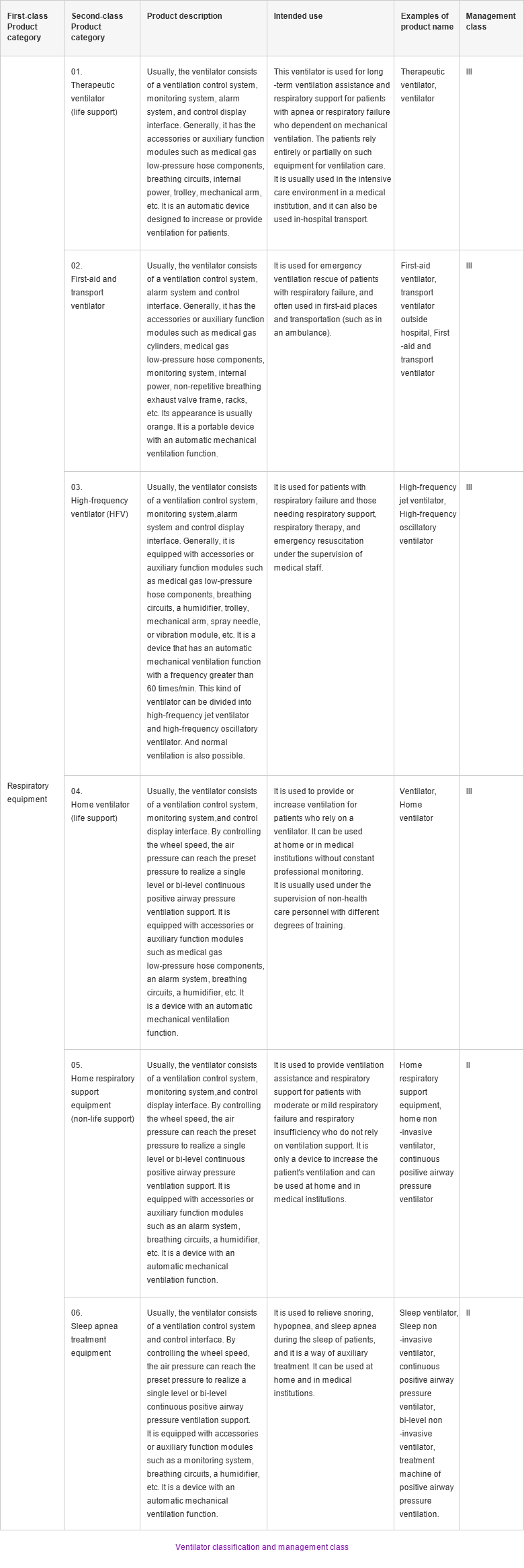
According to the classification standard of China medical devices, Class II medical devices are intermediate-risk devices, which require strict control and management to ensure their safety and effectiveness; Class III devices have higher risks, which need to be strictly controlled by special measures to ensure their safety and effectiveness.
Following the relevant provisions in China, medical devices in Class II, or Class III are subject to product registration management. To apply for registration of Class II medical device products, the applicant for registration shall submit registration application materials to the China Food and Drug Administration (CFDA)of the province, autonomous region, or municipality directly. To apply for registration of Class III medical device products, the applicant for registration shall submit registration application materials to the Food and Drug Administration of the State Council.
The application process typically takes a year or two. But the relevant departments may speed up the approval process during the current epidemic.
In addition to strict management standards, the ventilator supply chain is also very complicated. A ventilator is equivalent to a sophisticated industrial control system for gas delivery, which needs thousands of parts. If anyone of them fails, the entire device might not work.
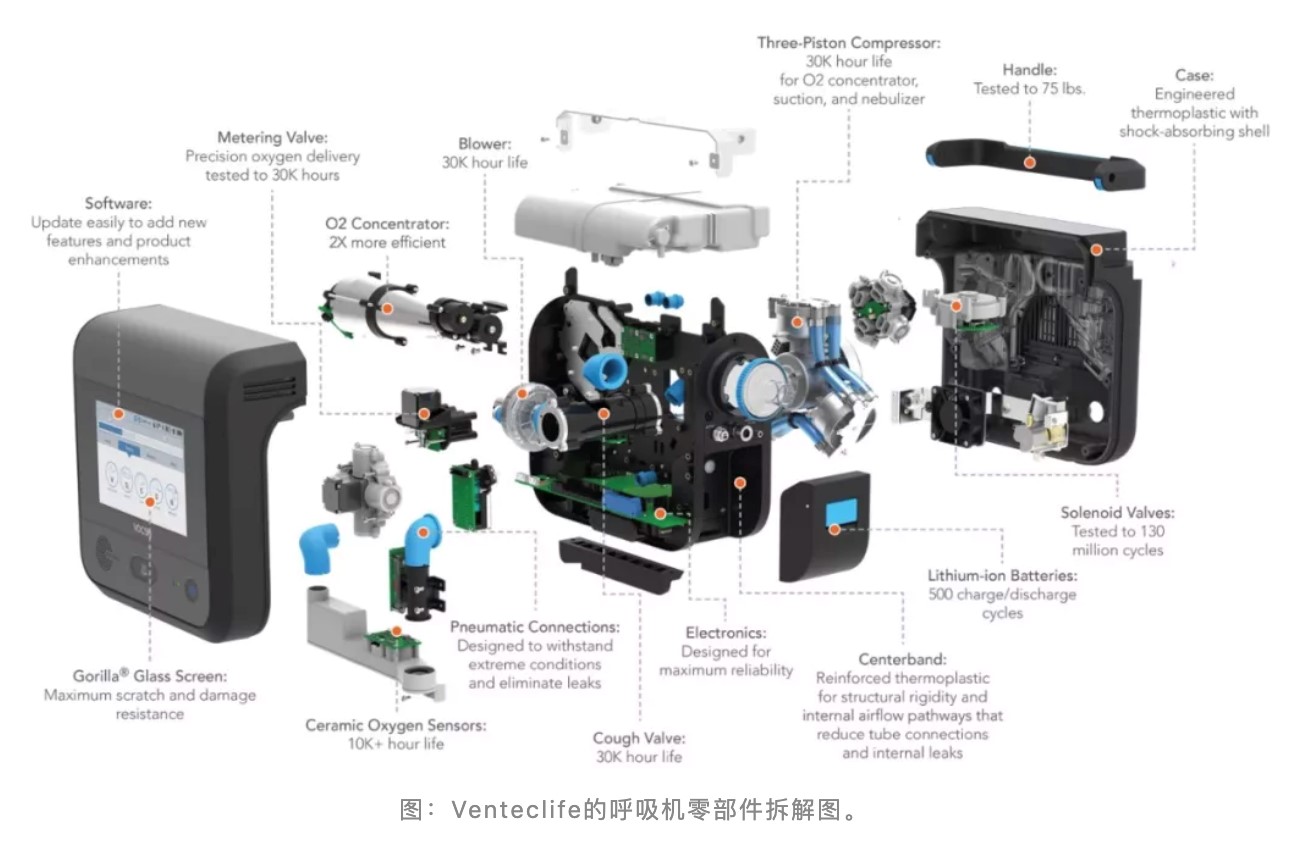
the parts disassembly diagram of a ventilator from Venteclife
Among these parts, the more critical are turbofans, sensors, chips, solenoid valves, oxygen sources, and so on. Some parts still rely on international suppliers distributed around the world.
For example, a responsible person in Yi'an revealed in an interview that the core components of the turbines are imported from Switzerland. And the proportional valves, proportional valve controllers, and sensors used by domestic ventilator manufacturers also need to be imported.
Peter Li, who comes from TE Sensors Division, told the Elecfans that the demand for ventilators' sensors has also surged recently and maybe as tight in the future as infrared sensors at the beginning of the year.
On April 7, in a telephone conversation with Cassis, the Swiss Federal Councilor and Foreign Minister, Chinese Foreign Minister Wang Yi also mentioned that he hoped Switzerland could significantly increase the supply of ventilator parts to help enterprises increase production and alleviate the urgent needs of other countries.
Also, according to market rumors, the order numbers of NXP' s pressure sensors currently are far higher than the original factory schedule, and the shortage will not be alleviated in Q2.
Will open-source solutions ease ventilator demand?
To meet the demand, Medtronic formally released the design document of the PB 560 ventilator on March 31, which includes manufacturing fixtures, printed circuit board (PCB) drawings, multiple bills of materials (BOM), and 3D CAD files.
On April 1, the company also released software source code files. Subsequently, the final document package of all information on other BOMs and equipment is also provided.
Could such an open-source solution alleviate the demand for ventilators? Maybe not, because the design of the PB 560 was completed more than ten years ago, and the devices are used are all ten years old. Now, if these devices are used to manufacture ventilators, how to buy these devices is also a problem. Verification will also take time if other chips are used instead.
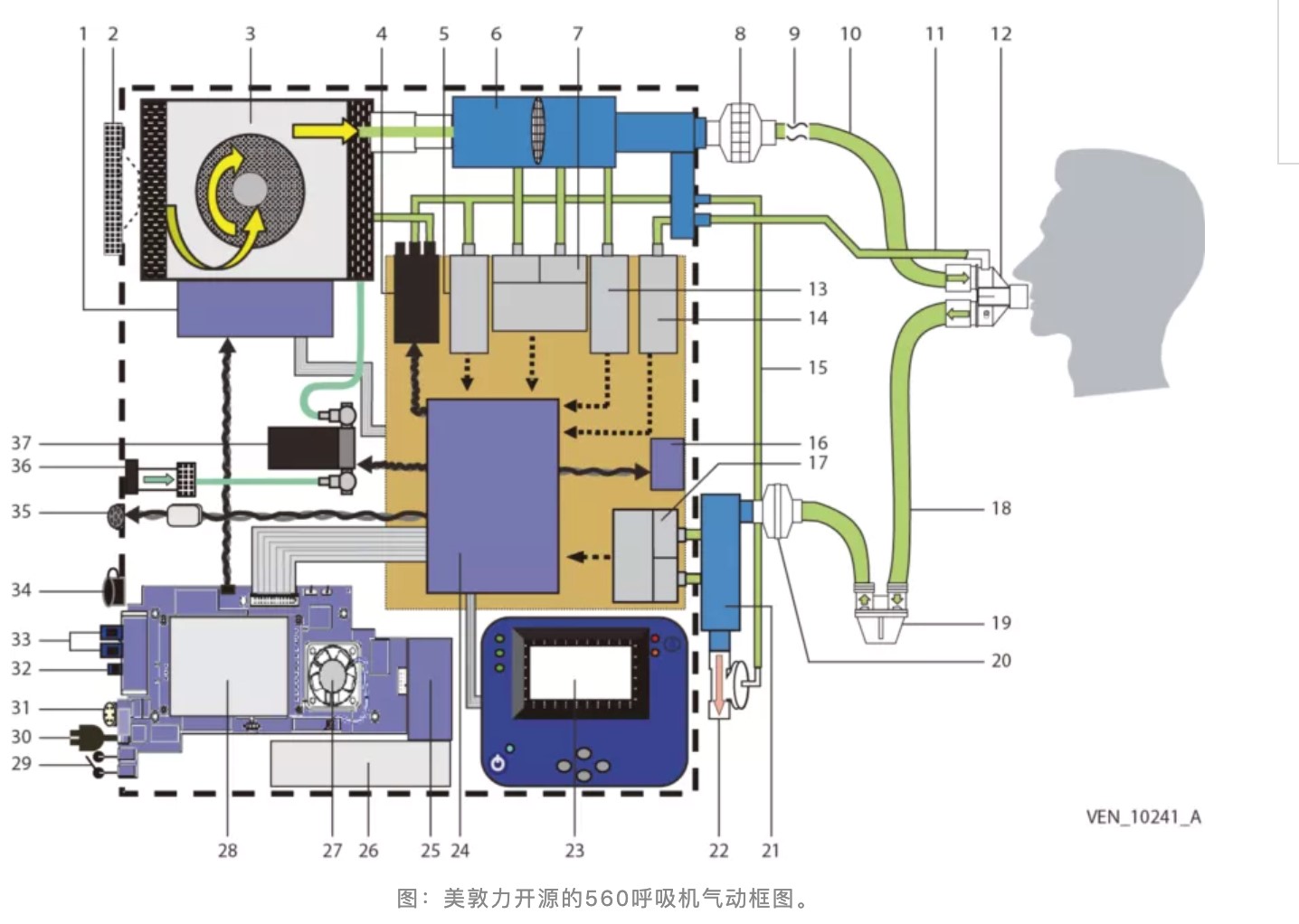
Pneumatic block diagram of Medtronic’s open-source 560 ventilator
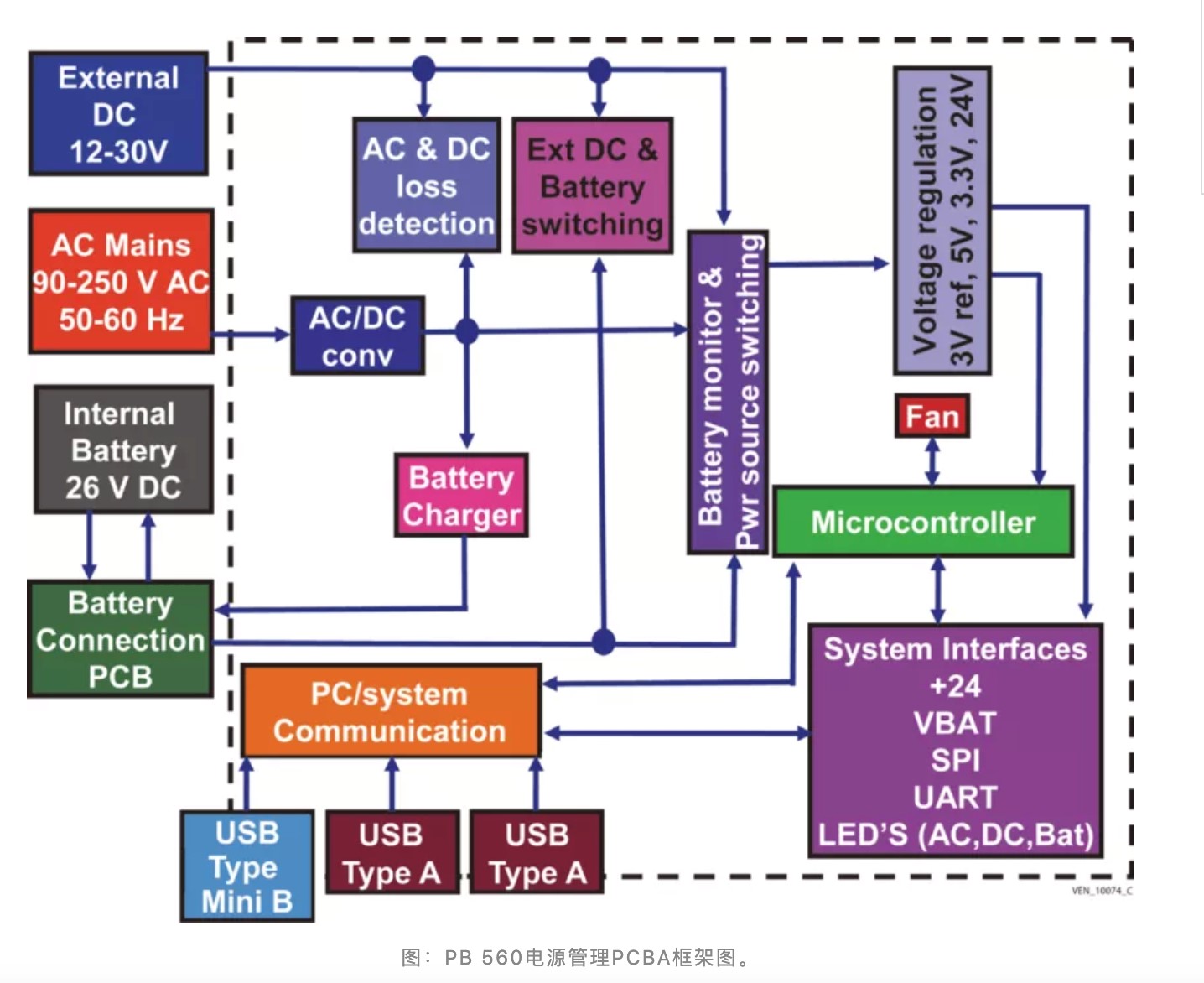
the power management PCBA block diagram of PB 560
In addition to the open-source solutions from traditional ventilator manufacturers, some hands-on netizens began to make their ventilators and open source them. For example, Johnny Lee' s solution in California, USA, is to convert a low-cost CPAP (continuous positive airway pressure ventilation) blower into an essential ventilator.

the physical map of Johnny Lee' s solution
Necessary materials in this solution can be brought from the internet, including CPAP blower ($ 20), Arduino Nano/Clone development board ($ 28), brushless DC motor speed controller, control switch, 12V DC power supply capable of handling 5A, Hall magnetic sensor, face mask, pipeline, and air purification device, etc. And the 3D printed drawings of blowers, face masks, and air purification devices are provided.
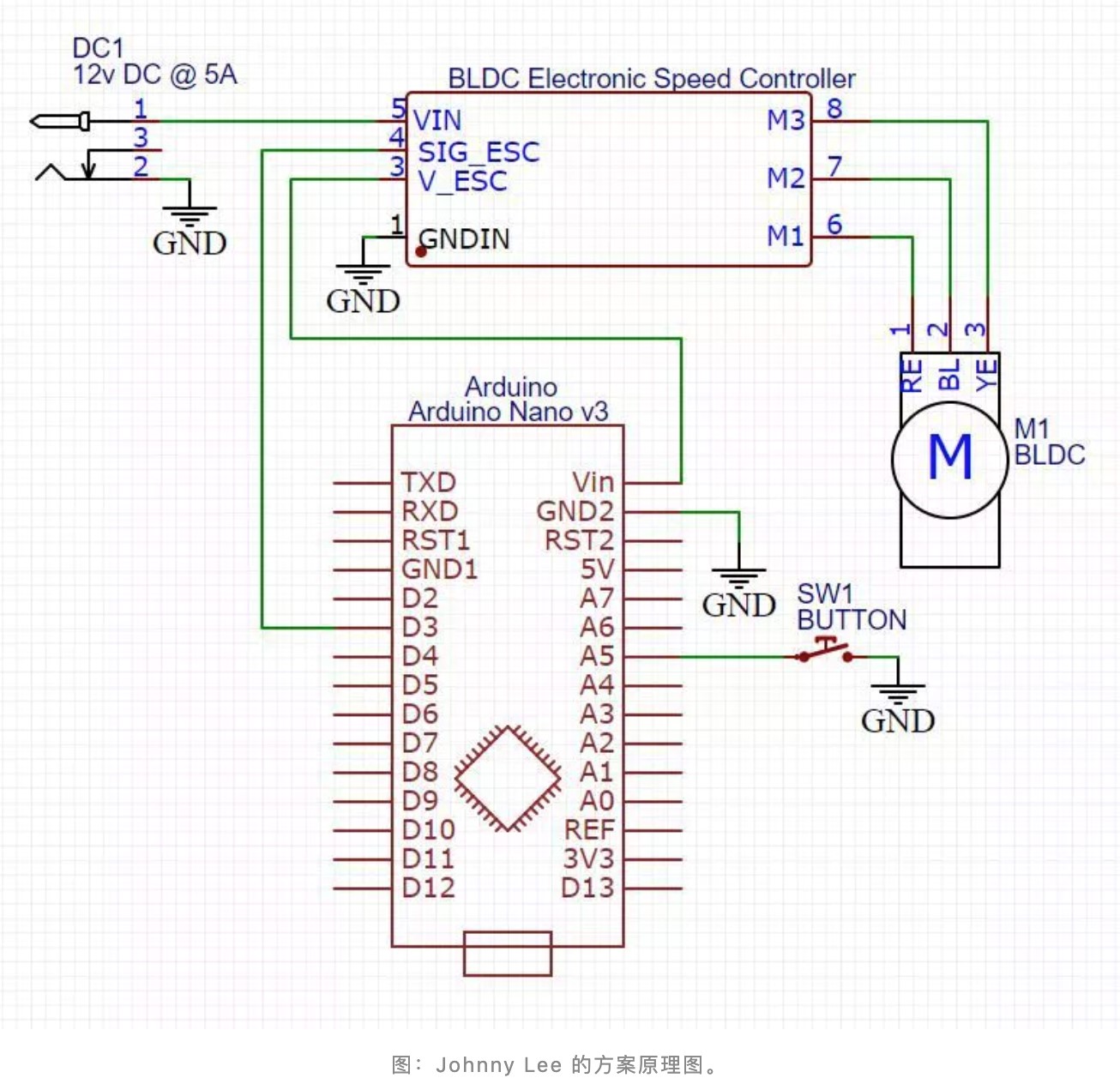
Johnny Lee’s solution schematic
Besides, the Coventor ventilator developed and designed by the University of Minnesota has got the authorization of the US Food and Drug Administration (FDA) for production. This project is trying to build a ventilator that can provide the same level of life-saving care capabilities with existing ventilators but at a much lower cost. It helps in achieving the goal of rapidly expanding production and making ventilators affordable for health facilities that need the devices.

The Coventor ventilator became the first new ventilator to be approved by the FDA's Emergency Use Authorization (EUA). The EUA, as its name, it is not a full approval of the traditional medical device, but a temporary authorization for emergency use.
The U of M' s Coventor developed by the team is about the size of a desktop computer and costs about 1,000 US dollars to manufacture. Compared with the price of 20,000 to 25,000 US dollars for a traditional ventilator, this can greatly reduce the burden of many medical institutions.
It is reported that the medical equipment manufacturers Medtronic and Boston Scientific participated in the design and development of this ventilator. Besides, the University of Minnesota announced on April 16 that they would open-source the specification data of Coventor so that it can start production globally.
Solutions from semiconductor manufacturers
At present, many semiconductor manufacturers and electronics industry chain manufacturers have opened green channels for medical equipment manufacturers. Many manufacturers have their ventilator solutions, such as NXP, Microchip, Maxim, Renesas, and so on.
The CPAP and ventilators of ST
A continuous positive airway pressure ventilator (CPAP) helps patients breathe by keeping the alveoli open and preventing the alveoli from collapsing completely during the expiratory phase. The most important thing about the CPAP system is the need to regulate airflow control to compensate for height, mask movement, and leakage, as well as heating, humidification, and airway respiratory support.
According to ST' s official website, ST' s motor control integrated circuit can help implement exact motion profiles, reduce acoustic noise, and improve patients' comfort. Besides, ST also provides a series of MEMS motion, pressure and humidity sensors, high-performance STM32 microcontrollers, high-precision operation amplifiers, and low-power regulators to help develop advanced CPAP ventilators.
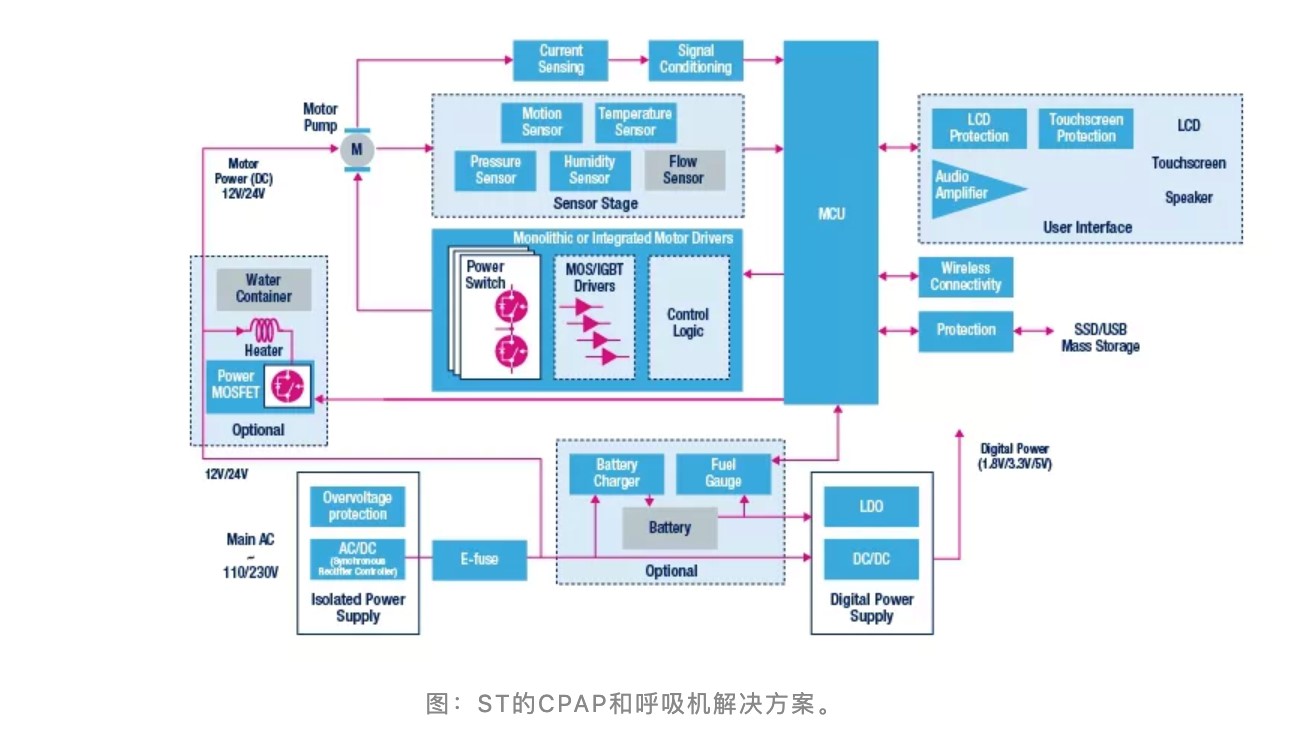
the solutions for ST’s CPAP and ventilators
The CPAP solution of Maxim
Maxim' s CPAP solution involves a small fan that generates a certain positive air pressure, which is introduced into the mouth of a patient with sleep apnea, keeping the airway open and relieving or eliminating the apnea symptoms.
The ventilator includes an adjustable air pressure fan, and a microcontroller in an embedded system controls the air pressure change. The embedded system contains air quality monitoring sensors and motor control sensors.
The mask pressure is monitored by the input sensor, which measures temperature, humidity, and pressure. Related sensors include motor winding current input detection, motor voltage detection, and temperature sensors.
The system output includes motor speed, buzzer alarm, or voice alarm.
The user interface mainly includes the switch (on/off), pressure control buttons, and pressure indication. For the more expensive system, the system indications can be achieved using backlit LEDs, and lower-priced systems can use simple signal LEDs.
Some CPAP ventilators are battery-powered portable designs, but most are grid-powered (bedside outlets). Grid-powered devices can also include battery backups for regular operation during power outages.
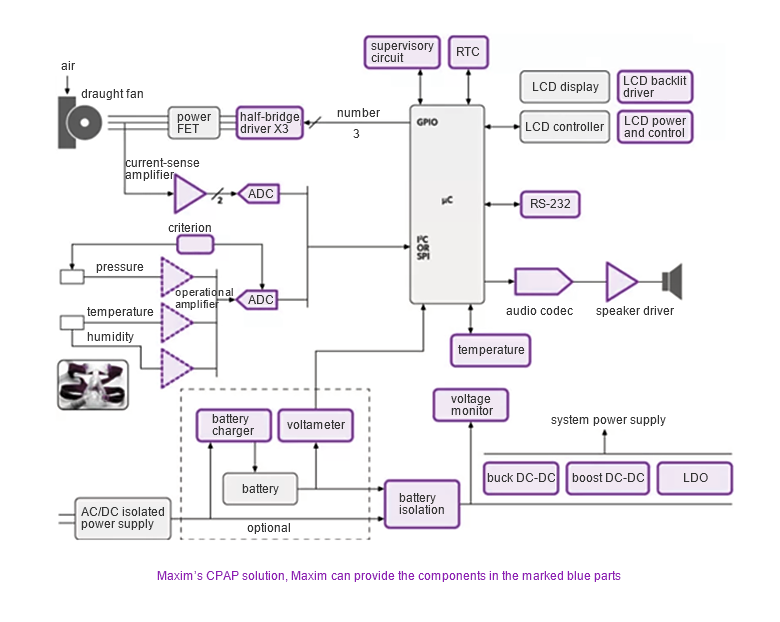
Maxim' s CPAP solution, Maxim can provide the components in the marked blue parts
The ventilator solution of NXP
This reference design of NXP is used to treat sleep apnea syndrome to prevent muscle clogging in the respiratory system. This is because CPAP can be combined with continuous monitoring of system pressure and motor speed control of a ventilator to maintain constant airflow. NXP can provide chips of MCU power management, motor drive, pressure sensors for this solution.
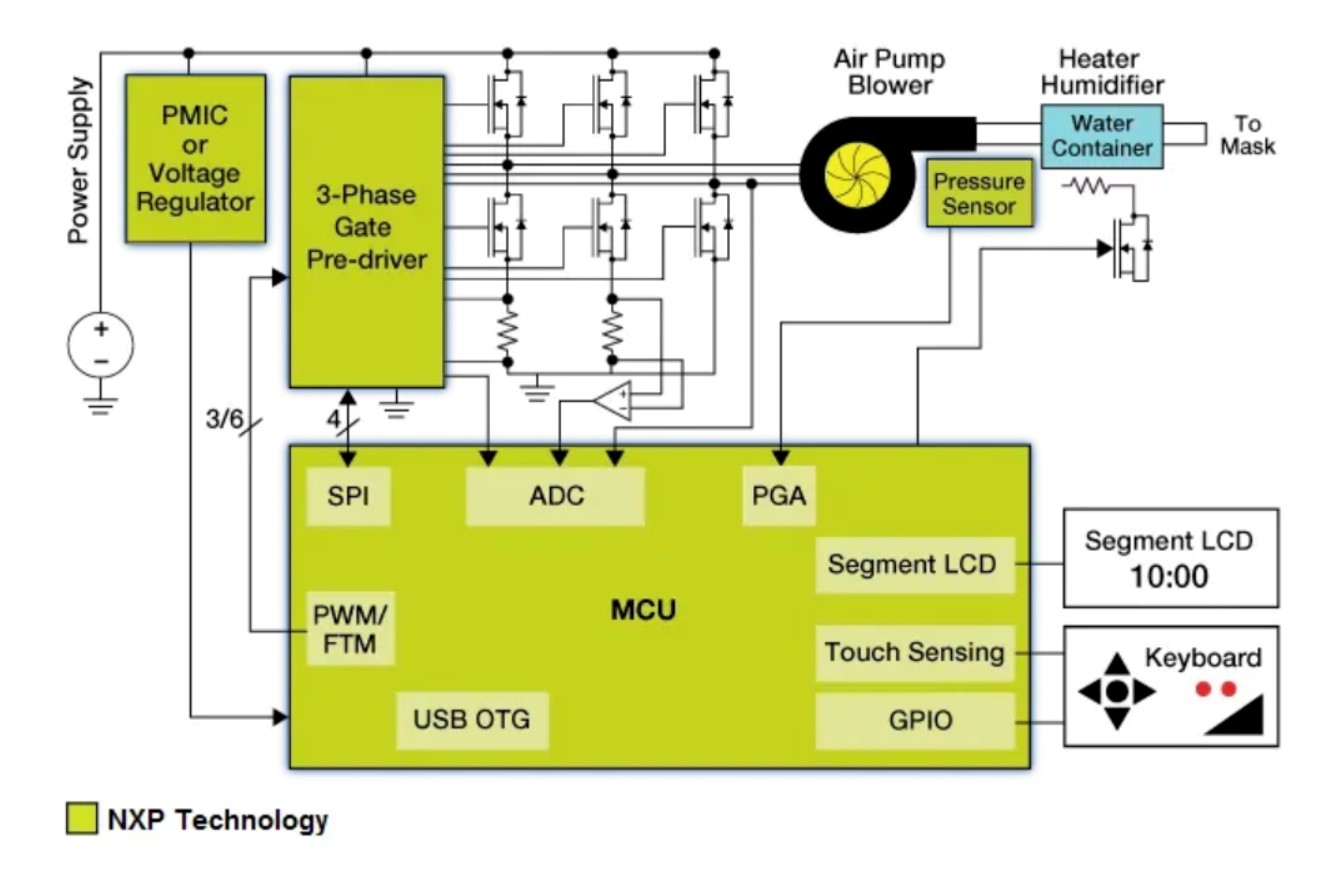
The ventilator solution of Microchip
Microchip' s ventilator solution adopts a dsPIC digital signal controller, which can implement multi-motor control strategies, including non-inductive BLDC control.
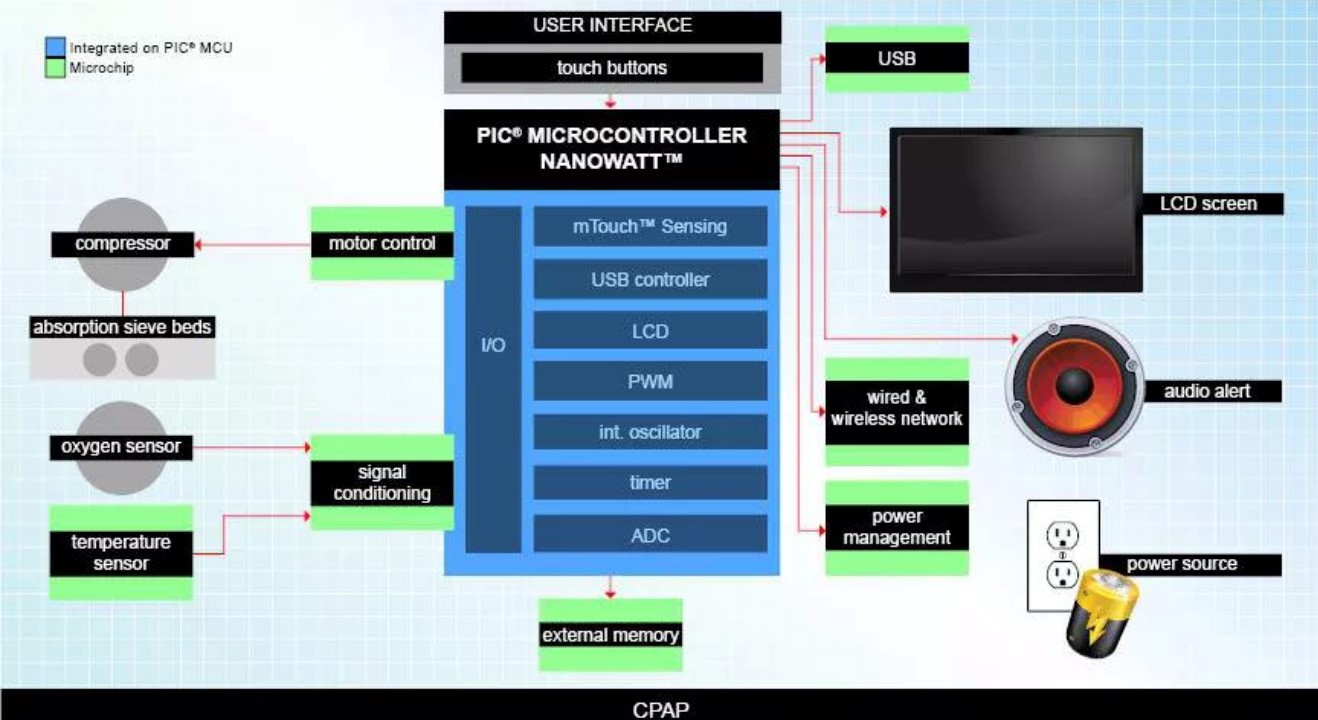
The medical ventilator solution of TI
In this medical ventilator solution, TI mainly provides motor drivers and power management chips.
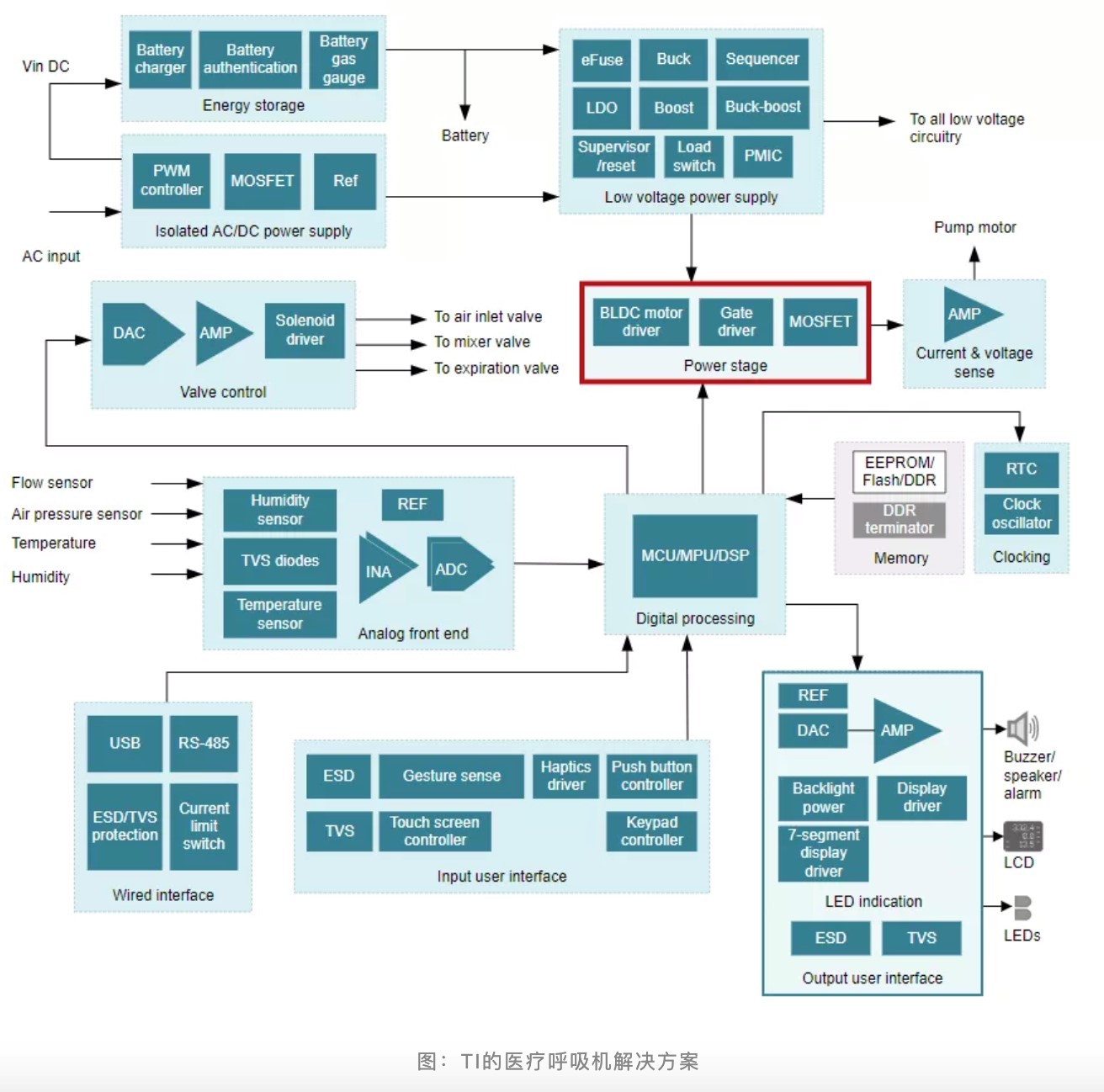
TI' s medical ventilator solution
Except for the ventilator solutions provided by these large semiconductor manufacturers, some domestic semiconductor enterprises also provide some reference solutions, such as Anlu Technology. But their solutions are still only at the negotiation stage and have not been used in mass production products.
Still, need help? Contact Us: support@nextpcb.com
Need a PCB or PCBA quote? Quote now
Still, need help? Contact Us: support@nextpcb.com
Need a PCB or PCBA quote? Quote now